May 23, 2018 report
What really causes Alzheimer's and how might we fix it?
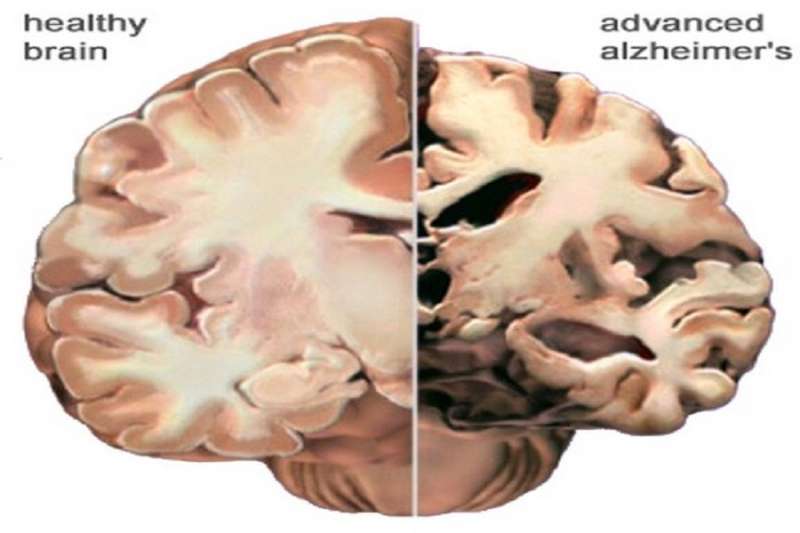
There have been a lot of theories about what causes Alzheimer's disease. Many of them have given rise to experimental treatments of one form or another. None of them have worked much better than taking anything you might find in your spice rack.
Medications to treat acetylcholine deficiency, vaccines that clear the amyloid plaques, and molecular chaperones that remove hyperphosphorylated tau were all potential therapies that failed to stop the disease. Researchers are now looking at mitochondrial function to find a possible root cause of all the pathologies that are part of Alzheimer's. In particular, the respiratory complexes involved in the electron transport chain have become a major focus.
Although pathologies like neuritic plaques and neurofibrillary tangles have been correlated with Alzheimer's, neither has proven to be causal. One way to demonstrate causality is taking something known to be correlated with a disease and then actually creating the disease with it. Another way is by showing you can prevent the disease by fiddling with that known factor.
A paper just published in bioRxiv now suggests researchers needn't look any further than the NADH dehydrogenase of respiratory complex I to find actionable targets. The authors were able were able to significantly delay the onset of Alzheimer's symptoms in an animal model by knocking down expression of the NDUFA9 subunit of complex I by RNA interference (RNAi). They were able to pinpoint NDUFA9 as a critical culprit in Alzheimer's by a meta-analysis of multi-dimensional human 'omic' data from 2000 autopsied human brains. This data was recently made available by Sage Bionetworks.
Their animal model was a transgenic worm line engineered to develop Alzheimer's-like pathology in the muscle cells of the body wall at a specific stage in their life cycle. Conveniently, these changes were associated with a readily observable paralysis of the worm. Since these worms normally feed on whole bacteria, it was possible to knock down NDUFA9 expression with RNAi by feeding them bacteria that deliver the RNAi.
The researchers were able to show that the RNAi feedings strongly decreased whole animal oxygen consumption, reduced amyloid-beta plaque toxicity, and significantly delayed any paralysis. In other words, a home run. Further experiments revealed that knockdown of any of 13 additionally tested complex I subunits also delayed paralysis. This clearly identifies the NADH dehydrogenase as the culprit.
The big question is how all this works, and therefore by implication, how might we implement a similar 'therapeutic' strategy in humans? The authors suggest that mild complex I inhibition could be protective in humans. It is important to realize that complex I is essential in humans. When it is genetically broken, as in several mitochondrial disorders, the results are devastating. There are several known variants in complex I subunits associated with LHONs syndrome and MELAS syndrome that are associated with very specific and damaging pathology. In Leigh's syndrome, the NDUFA9 subunit is one of many that can be mutated.
It appears that Alzheimer's patients may already have reduced complex I expression to begin with. This could reflect some compensatory mechanism that has naturally been initiated in the body. Drugs like capsaicin, or the small molecule CP2, might be given to further inhibit complex I. If so, it will be important to have some measure of baseline NADH dehydrogenase activity to set the proper dose.
There are several major differences between our mitochondria, and those of worms like c. Elegans. We just reported how succinate dehydrogenase (complex II) can run in reverse under certain conditions to regenerate the quinone electron carrier pool for use in complex I. While this has been shown to occur in the low-oxygen environments found in several kinds of human tumors, it is not known if this mini-cycle can also be a good thing for us.
Worms have special versions of complex II subunits that they can swap in as needed. They also have an alternative quinone electron carrier called rhodoquinone designed to work with the special complex II. Although these factors complicate the extrapolation of results in worms to humans somewhat, they do provide us with a much clearer understanding of respiratory chain activity in disease states.
More information: Safiye Celik et al. A probabilistic approach to using big data reveals Complex I as a potential Alzheimer's disease therapeutic target, (2018). DOI: 10.1101/302737
Abstract
Identifying gene expression markers for Alzheimer's disease (AD) neuropathology through meta-analysis is a complex undertaking because available data are often from different studies and/or brain regions involving study-specific confounders and/or region-specific biological processes. Here, we developed a probabilistic model-based framework, DECODER, leveraging these discrepancies to identify robust biomarkers for complex phenotypes. Our experiments present: (1) DECODER's potential as a general meta-analysis framework widely applicable to various diseases (e.g., AD and cancer) and phenotypes (e.g., Amyloid-β; (Aβ) pathology, tau pathology, and survival), (2) our results from a meta-analysis using 1,746 human brain tissue samples from nine brain regions in three studies—the largest expression meta-analysis for AD, to our knowledge —, and (3) in vivo validation of identified modifiers of Aβ toxicity in a transgenic Caenorhabditis elegans model expressing AD-associated Aβ, which pinpoints mitochondrial Complex I as a critical mediator of proteostasis and a promising pharmacological avenue toward treating AD.
© 2018 Medical Xpress